Why Should've You Attend the 7th Complement-Based Drug Development Summit
Returning for its 7th year, the Complement-Based Drug Development Summit evaluated the entirety of the complement landscape, from scientific understanding to strategic, commercial intelligence.
With the success of the first and only treatment for Geographic Atrophy, a complement-based therapeutic, this expanding industry is heating up.
This summit returned in a timely fashion to delve into the expanding therapeutic opportunities: from understanding the degree of complement inhibition needed, to mitigating toxicity effects with targeted approaches, participants heard about how clinical scientists are optimizing clinical development by uncovering predictive biomarkers better informing patient populations, interpreting results, and strategizing regulatory approval across ophthalmology, neurology, haematology, and nephrology.
New for 2023, a full pre-conference workshop composed of 4 deep diving discursive sessions split into a preclinical and clinical stream, both dedicated to overcoming the bottlenecks, and progressing development no matter what stage your pipeline is.
Participants Networked with 100+ industry colleagues from around the globe, joining the largest community of complement-based drug development experts to build meaningful connections and gather insights beyond the literature.
The 2023 Interactive Discussions Featured:
A dive into preclinical modelling of the complement system, exploring current models and evaluating room for improvement with AGTC, Moderna, The National Institutes of Health and Boston University
An Exploration of the most beneficial target whether proximal or terminal and uncovered the scientific rationale behind which fragment to target for which diseases with Sirnaomics and Annexon
Strategies to achieve regulatory approval and to overcome commercial challenges to drive the next generation of complement therapeutics with experts from Sanofi and UCB
A Recap of how the complement landscape has evolved over the years and explore where future opportunities and commercial success lie with Alexion, Apellis, Annexon and InflaRx
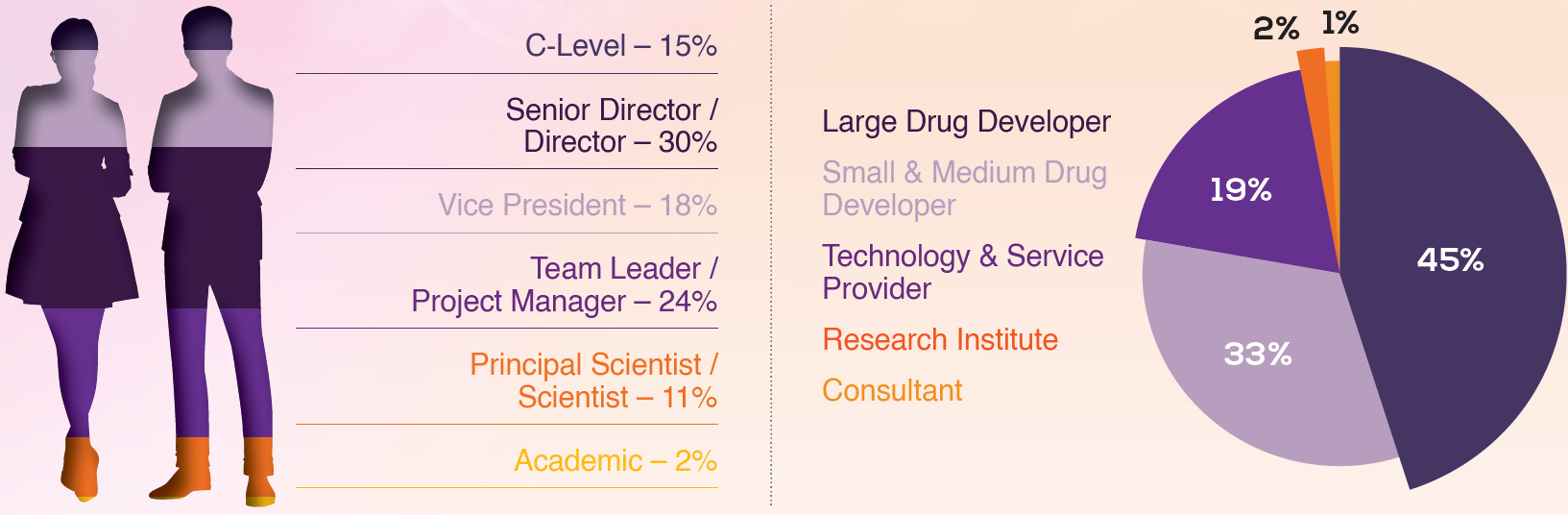
2023 Attendees
With 100+ attendees from around the globe, this is your community of complement-based drug developers spanning nephrology, neurology, ophthalmology, and haematology.
What Your Peers Have to Say:
“Multiple high-quality speakers and an introduction to new and emerging startups and biotech drug development programs.” UCB
“The strong scientific meeting agenda with such a remarkable expert panel. ” Q32
“I enjoyed the content from all the presentations; was pleased to see the ongoing research and complement-based drug development from various companies. ” Omeros
